Research
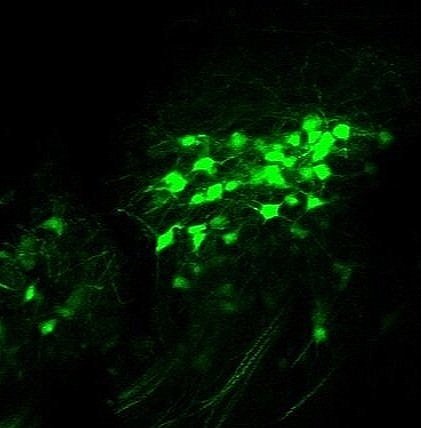
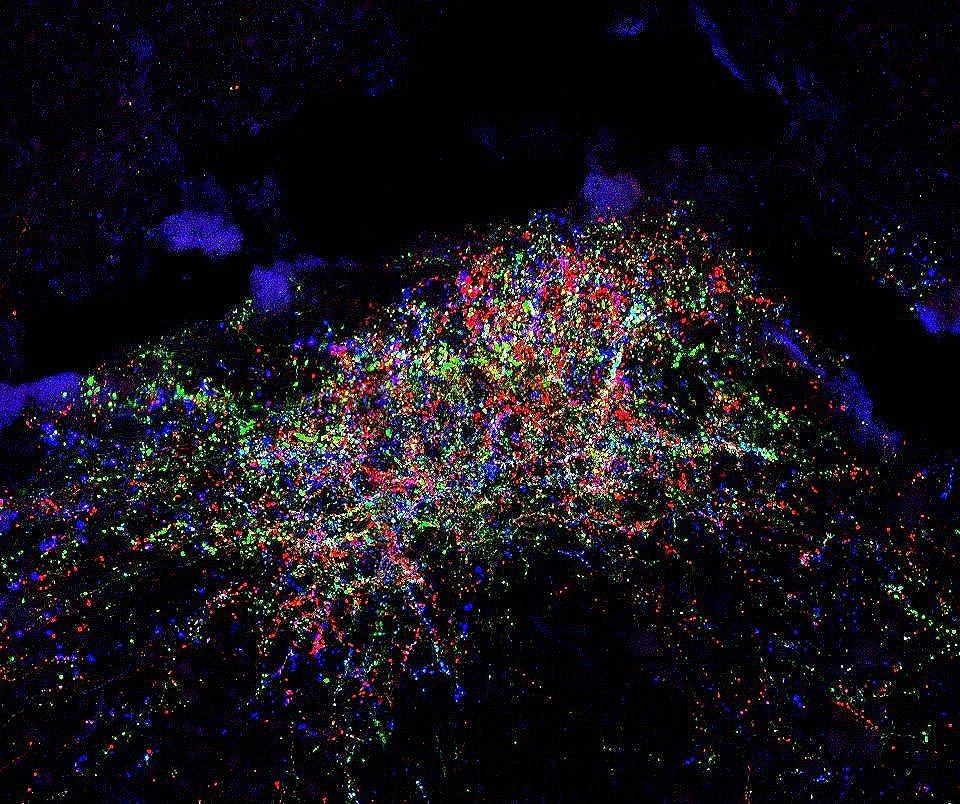
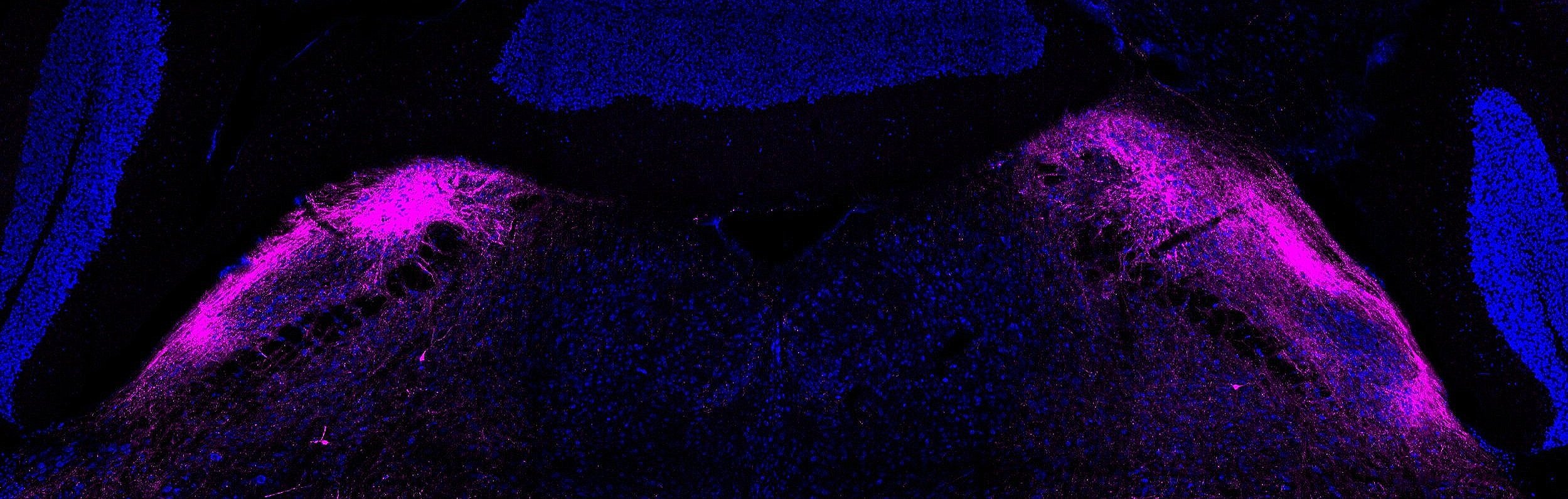
Each day we experience myriad somatosensory stimuli: hugs from loved ones, warm showers, a mosquito bite, sore muscles after a workout. These tactile, thermal, itch, and nociceptive signals are detected by peripheral sensory neuron terminals and end organs distributed throughout our body, propagated into the spinal cord where they are processed, and transmitted to the brain via ascending spinal projection pathways. Primary sensory neurons that innervate the skin and detect a wide range of somatosensory stimuli have been identified and characterized. In contrast, very little is known about how peripheral signals are integrated and processed within the spinal cord and how these signals are conveyed to the brain by spinal projection neurons to generate somatosensory perception and behavioral responses. Our lab aims to determine the developmental logic and functional organization of ascending somatosensory circuitry and to use this knowledge to reveal how internal states and disorders of the nervous system shape our sense of touch and pain. Our lab explores these exciting areas using new mouse genetic tools in conjunction with advanced molecular, anatomical, physiological, and behavioral approaches.
Functional organization of ascending somatosensory circuitry
The perception of touch and pain is multidimensional; upon touch or pinch of our skin, we discriminate between a gentle stroke and a sharp pinch, turn our body and head towards the stimuli, and evaluate hedonic values associated with them (pleasant vs. hurting). Moreover, how we perceive and react to different sensory cues is influenced by the nature of sensory stimuli; touching a hot pan and caressing a dog give rise to different sensations and behavioral responses. Our lab is interested in studying how ascending somatosensory circuitry endows us with this complex sensation of touch and pain and reaction to them.
Dysfunction of ascending somatosensory circuitry
Touch and pain are subjective experiences that are greatly modulated by internal states as well as pathological conditions: a gentle touch can be perceived as painful or disturbing in people with neuropathic pain or autism spectrum disorders. Our lab is interested in studying how disease states shape our sense of touch and pain and what molecular, cellular, and circuit mechanisms underlie the dysfunction of ascending somatosensory circuitry. This may reveal new therapeutic strategies for treating disorders associated with touch and pain.
Development of ascending somatosensory circuitry
Ascending spinal pathways consist of multiple independent modules that convey touch and pain signals to many different regions in the brain, including the brainstem, pons, midbrain, and thalamus. The sophisticated structural connectivity enables precise propagation of somatosensory signals from the periphery to the brain, underlying our sensation of and reaction to touch and pain. Our lab is interested in studying how these complex ascending somatosensory circuits are wired during development and what developmental programs control their formation and organization.
Experimental approaches
-
Mouse genetics

-
Anatomy/Viral tracing

-
Slice physiology

-
Behavior/Optogenetics

-
Multielectrode array (MEA) recordings

-
Fiberphotometry/Miniscope imaging